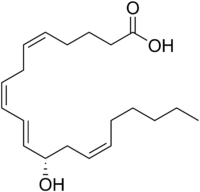
12-Hydroxyeicosatetraenoic acid

12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a Hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiquous in that it has been used to indicate not only the initially detected 'S' stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected 'R' stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances. 12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a Hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiquous in that it has been used to indicate not only the initially detected 'S' stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected 'R' stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances. In humans, Arachidonate 12-lipoxygenase (12-LO, 12-LOX, ALO12, or platelet type 12-lipoxygenase) is encoded by the ALOX12 gene and expressed primarily in platelets and skin. ALOX12 metabolizes arachidonic acid almost exclusively to 12(S)-hydroperoxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HpETE or 12S-HpETE). Arachidonate 15-lipoxygenase-1 (15-LO-1, 15-LOX-1, ALOX15), which is expressed in far more tissues that ALOX12, metabolizes arachidonic acid primarily to 15(S)-HpETE along with other metabolites of the 15-Hydroxyicosatetraenoic acid family; during this metabolism, however, ALOX15 also forms 12(S)-HpETE as a minor product. Arachidonate 12-lipoxygenase, 12R type, also termed 12RLOX and encoded by the ALOX12B gene, is expressed primarily in skin and cornea; it metabolizes arachidonic acid to 12(R)-HpETE. Cytochrome P450 enzymes convert arachidonic acid to a variety of hydroperoxy, epoxy, and dihydroxy derivatives including racemic mixtures of 12(S)-HpETE and 12(R)-HpETE or 12(S)-HETE and 12(R)-HETE; the R stereoisomer predominates in these mixtures. The initial 12(S)-HpETE and 12(R)-HpETE products, regardless of their pathway of formation, are rapidly reduced to 12(S)-HETE and 12(R)-HETE, respectively, by ubiquitous cellular peroxidases, including in particular Glutathione peroxidases or, alternatively, are further metabolized as described below. Sub-primate mammals, such as the mouse, rat, rabbit, cow, and pig, express platelet type 12-lipoxygenase but also a leukocyte type 12-lipoxygenase (also termed 12/15-lipoxygenase, 12/15-LOX or 12/15-LO) which is an ortholog of, and metabolically equivalent to, human 15-LO-1 in that it forms predominantly 15(S)-HpETE with 12(S)-HpETE as a minor product. Mice also express an epidermal type 15-lipoxygenase (e-12LO) which has 50.8% amino acid sequence identity to human 15-LOX-2 and 49.3% sequence indetity to mouse Arachidonate 8-lipoxygenase. Mouse e-12LO metabolizes arachidonic acid predominantly to 12(S)-HETE and to a lesser extent 15(S)-HETE. Sub-human primates, although not extensively examined, appear to have 12-lipoxygenase expression patterns that resemble those of sub-primate mammals or humans depending on the closeness of there genetic relateness to these species. In human (and mouse) skin epidermis, 12(R)-HpETE is metabolized by Epidermis-type lipoxygenase, i.e. eLOX3 (encoded by the ALOXE3 gene), to two products: a) a specific hepoxilin, 8R-hydroxy-11R,12R-epoxy-5Z,9E,14Z-eicosatetraenoic acid (i.e. 8R-hydroxy,11R,12R-epoxy-hepoxilin A3 or 8R-OH,11R,12R-epoxy-hepoxilin A3) and b) 12-oxo-5Z,8Z,10E,14Z-eicosatetraenoic acid (12-oxo-HETE, 12-oxoETE, 12-Keto-ETE, or 12-KETE); 8R-hydroxy,11R,12R-epoxy-hepoxilin A3 is further metabolized by soluble Epoxide hydrolase 2 (sEH) to 8R,11R,12R-trihydroxy-5Z,9E,14Z-eicosatetraenoic acid. 12(R)-HpETE also spontaneously decomposes to a mixture of hepoxilins and trihydroxy-eicosatetraenoic acids that possess R or S hydroxy and epoxy residues at various sites while 8R-hydroxy,11R,12R-epoxy-hepoxilin A3 spontaneously decomposes to 8R,11R,12R-trihydroxy-5Z,9E,14Z-eicosatetraenoic acid. These decompositions may occur during tissue isolation procedures. Recent studies indicate that the metabolism by ALOXE3 of the R stereoisomer of 12-HpETE made by ALOX12B and therefore possibly the S stereoisomer of 12-HpETE made by ALOX12 or ALOX15 is responsible for forming various hepoxilins in the epidermis of human and mouse skin and tongue and possibly other tissues. Human skin metabolizes 12(S)-HpETE in reactions strictly analogous to those of 12(R)-HpETE; it metabolized 12(S)-HpETE by eLOX3 to 8R-hydroxy-11S,12S-epoxy-5Z,9E,14Z-eicosatetraenoic acid and 12-oxo-ETE, with the former product then being metabolized by sEH to 8R,11S,12S-trihydroxy-5Z,9E,14Z-eicosatetraenoic acid. 12(S)-HpETE also spontaneously decomposes to a mixture of hepoxilins and trihydroxy-eicosatetraenoic acids (trioxillins) that possess R or S hydroxy and R,S or S,R epoxide residues at various sites while 8R-hydroxy,11S,12S-epoxy-hepoxilin A3 spontaneously decomposes to 8R,11S,12S-trihydroxy-5Z,9E,14Z-eicosatetraenoic acid. In other tissues and animal species, numerous hepoxilins form but the hepoxilin synthase activity responsible for their formation is variable. (Hepoxilin A3 and hepoxilin B3 refer to a mixture of Diastereomers and⁄or Enantiomers derived from arachidonic acid.) Cultured RINm5F rat Insulinoma cells convert 12(S)-HpETE to hepoxilin A3 in a reaction that is completely dependent on, and co-localizes with, the cells' leukocyte type 12-LOX; furthermore, recombinant rat and porcine leukocyte type 12-LOX as well as human platelet type 12-LOX metabolize 12(S)-HpETE to hepoxylin A3. However, transfection of HEK293 human embryonic kidney cells with each of the 6 rat lipoxygenases, including rat eLOX3, found that hepoxilin B3 production required eLOX3; furthermore, the development of inflammation-induced tactile pain hypersensitivity (hyperesthesia; tactile Allodynia) in rats required eLOX3-dependent production of hepoxilin B3 by spinal tissue. Thus, the production of hepoxilins from 12(S)-HpETE may result from the intrinsic activity of platelet or leukocyte type 12-LOX's, require eLOX3, or even result from 12(S)-HpETE spontaneous (and perhaps artefactual) decomposition during isolation. The majority of reports on hepoxilin formation have not defined the pathways evolved. Human and other mammalian cytochrome P450 enzymes convert 12(S)-HpETE to 12-oxo-ETE. 12-HETE (stereoisomer not determined), 12(S)-HETE, 12-oxo-ETE, hepoxilin B3, and trioxilin B3 are found in the sn-2 position of phospholipids isolated from normal human epidermis and human psoriatic scales. This indicates that the metabolites are acylated into the sn-2 position after being formed and/or directly produced by the metabolism of the arachidonic acid at the sn-2 position of these phospholipids. These acylation reactions may sequester and thereby inactivate or store the metabolites for release during cell stimulation.