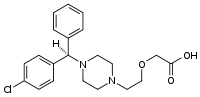
Levocetirizine

Levocetirizine, sold under the brand name Xyzal among others, is an antihistamine used for the treatment of allergic rhinitis (hay fever) and long term hives of unclear cause. It is less sedating than older antihistamines. It is taken by mouth. Levocetirizine, sold under the brand name Xyzal among others, is an antihistamine used for the treatment of allergic rhinitis (hay fever) and long term hives of unclear cause. It is less sedating than older antihistamines. It is taken by mouth. Common side effects include sleepiness, dry mouth, cough, vomiting, and diarrhea. Use in pregnancy appears safe but has not been well studied and use when breastfeeding is of unclear safety. It is classified as a second-generation antihistamine and works by blocking histamine H1-receptors. Levocetirizine was approved for medical use in the United States in 2007. It is available as a generic medication. A month supply in the United Kingdom costs the NHS about 4.50 £ as of 2019. In the United States the wholesale cost of this amount is about US$3. In 2016 it was the 163rd most prescribed medication in the United States with more than 3 million prescriptions. Levocetirizine is used for allergy symptoms including watery eyes, runny nose, sneezing, hives, and itching. The manufacturers claim it to be more effective with fewer side effects than previous second-generation drugs; however, there have been no published independent studies supporting these comparative assertions. A study part-funded by the manufacturer UCB concluded it may be more effective than some other second- and third-generation anti-histamines, but did not compare it to cetirizine. Levocetirizine is called a non-sedating antihistamine as it does not enter the brain in significant amounts, and is therefore unlikely to cause drowsiness. Cardiac safety with repolarization may be better than some other antihistamines, as levocetirizine does not significantly prolong the QT interval in healthy individuals. However, some people may still experience some slight sleepiness, headache, mouth dryness, lightheadedness, vision problems (mainly blurred vision), palpitations and fatigue. Levocetirizine is an antihistamine. It acts as an inverse agonist that decreases activity at histamine H1 receptors. This in turn prevents the release of other allergy chemicals and increase the blood supply to the area, and provides relief from the typical symptoms of hay fever. Chemically, levocetirizine is the active levorotary enantiomer of cetirizine, also called the l-enantiomer of cetirizine. It is a member of the diphenylmethylpiperazine group of antihistamines. Levocetirizine was first launched in 2001 by Belgian pharmaceutical company UCB. On 31 January 2017, the Food and Drug Administration approved an over-the-counter version. Although the drug was authorized by the FDA in 2007, it was already available in most European countries. Like many new drugs it entered the market at a higher price than currently available third and second generation antihistamines. In India, one form of the drug is available as Crohist MK tablets and syrup, a formulation of levocetirizine hydrochloride and montelukast. In India, Crohist MK is a Schedule 'H' drug and may only be prescribed by a registered medical practitioner.