Ammonium

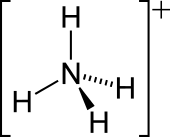
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4. It is formed by the protonation of ammonia (NH3). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (NR+4), where one or more hydrogen atoms are replaced by organic groups (indicated by R).The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors):Ammonium cation is found in a variety of salts such as ammonium carbonate, ammonium chloride and ammonium nitrate. Most simple ammonium salts are very soluble in water. An exception is ammonium hexachloroplatinate, the formation of which was once used as a test for ammonium. The ammonium salts of nitrate and especially perchlorate are highly explosive, in these cases ammonium is the reducing agent.The lone electron pair on the nitrogen atom (N) in ammonia, represented as a line above the N, forms the bond with a proton (H+). Thereafter, all four N–H bonds are equivalent, being polar covalent bonds. The ion has a tetrahedral structure and is isoelectronic with methane and borohydride. In terms of size, the ammonium cation (rionic = 175 pm) resembles the caesium cation (rionic = 183 pm).The hydrogen atoms in the ammonium ion can be substituted with an alkyl group or some other organic group to form a substituted ammonium ion (IUPAC nomenclature: aminium ion). Depending on the number of organic groups, the ammonium cation is called a primary, secondary, tertiary, or quaternary. With the exception of the quaternary ammonium cations, the organic ammonium cations are weak acids.Ammonium ions are a waste product of the metabolism of animals. In fish and aquatic invertebrates, it is excreted directly into the water. In mammals, sharks, and amphibians, it is converted in the urea cycle to urea, because urea is less toxic and can be stored more efficiently. In birds, reptiles, and terrestrial snails, metabolic ammonium is converted into uric acid, which is solid and can therefore be excreted with minimal water loss.The ammonium ion has very similar properties to the heavier alkali metals and is often considered a close relative. Ammonium is expected to behave as a metal (NH+4 ions in a sea of electrons) at very high pressures, such as inside gas giant planets such as Uranus and Neptune.