Unsymmetrical dimethylhydrazine

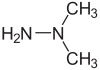
Unsymmetrical dimethylhydrazine (UDMH; 1,1-dimethylhydrazine) is a chemical compound with the formula H2NN(CH3)2. It is a colorless liquid, with a sharp, fishy, ammonia-like smell typical for organic amines. Samples turn yellowish on exposure to air and absorb oxygen and carbon dioxide. It mixes completely with water, ethanol, and kerosene. In concentration between 2.5% and 95% in air, its vapors are flammable. It is not sensitive to shock. 1,2-Dimethylhydrazine (CH3NHNHCH3) is also known but is not as useful. Unsymmetrical dimethylhydrazine (UDMH; 1,1-dimethylhydrazine) is a chemical compound with the formula H2NN(CH3)2. It is a colorless liquid, with a sharp, fishy, ammonia-like smell typical for organic amines. Samples turn yellowish on exposure to air and absorb oxygen and carbon dioxide. It mixes completely with water, ethanol, and kerosene. In concentration between 2.5% and 95% in air, its vapors are flammable. It is not sensitive to shock. 1,2-Dimethylhydrazine (CH3NHNHCH3) is also known but is not as useful. UDMH is produced industrially by two routes. One, based on the Olin Raschig process, involves reaction of chloramine with dimethylamine. This method gives the hydrochloride of the hydrazine: Alternatively, acetylhydrazine can be N-methylated using formaldehyde to give the N,N-dimethyl-N'-acetylhydrazine, which can subsequently be hydrolyzed: UDMH is often used in hypergolic rocket fuels as a bipropellant in combination with the oxidizer nitrogen tetroxide and less frequently with IRFNA (red fuming nitric acid) or liquid oxygen. UDMH is a derivative of hydrazine and is sometimes referred to as a hydrazine. As a fuel, it is described in specification MIL-PRF-25604 in the United States. UDMH is stable and can be kept loaded in rocket fuel systems for long periods, which makes it appealing for use in many liquid rocket engines, despite its cost. In some applications, such as the OMS in the Space Shuttle or maneuvering engines, monomethylhydrazine is used instead due to its slightly higher specific impulse.In some kerosene-fueled rockets, UDMH functions as a starter fuel to start combustion and warm the rocket engine prior to switching to kerosene.UDMH has higher stability than hydrazine, especially at elevated temperatures, and can be used as its replacement or together in a mixture. UDMH is used in many European, Russian, Indian, and Chinese rocket designs. The Russian Proton, Kosmos-3M, and the Chinese Long March 2F are the most notable users of UDMH (which is referred to as 'heptyl' by Russian engineers). The Titan, GSLV, and Delta rocket families use a mixture of 50% hydrazine and 50% UDMH, called Aerozine 50, in different stages. There is speculation that it is the fuel used in the ballistic missiles that North Korea has developed and tested in 2017. Apart from its use as rocket fuel, UDMH is a nitrogen source in metalorganic vapor phase epitaxy thin-film deposition. UDMH is a contaminant, metabolite, and breakdown product of daminozide. Hydrazines and its methyl derivatives are toxic but LD50 values have not been reported.(This part needs edit with data provided by reference) Hydrazines are classified as carcinogens.