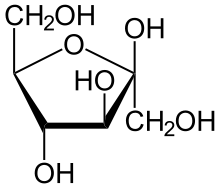
Fructose

Fructose, or fruit sugar, is a simple ketonic monosaccharide found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into blood during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847. The name 'fructose' was coined in 1857 by the English chemist William Allen Miller. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars.Fructose is found in honey, tree and vine fruits, flowers, berries, and most root vegetables. Fructose, or fruit sugar, is a simple ketonic monosaccharide found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into blood during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847. The name 'fructose' was coined in 1857 by the English chemist William Allen Miller. Pure, dry fructose is a sweet, white, odorless, crystalline solid, and is the most water-soluble of all the sugars.Fructose is found in honey, tree and vine fruits, flowers, berries, and most root vegetables. Commercially, fructose is derived from sugar cane, sugar beets, and maize. Crystalline fructose is the monosaccharide, dried, ground, and of high purity. High-fructose corn syrup is a mixture of glucose and fructose as monosaccharides. Sucrose is a compound with one molecule of glucose covalently linked to one molecule of fructose. All forms of fructose, including fruits and juices, are commonly added to foods and drinks for palatability and taste enhancement, and for browning of some foods, such as baked goods. About 240,000 tonnes of crystalline fructose are produced annually. Excessive consumption of fructose may contribute to insulin resistance, obesity, elevated LDL cholesterol and triglycerides, leading to metabolic syndrome. The European Food Safety Authority stated that fructose is preferable over sucrose and glucose in sugar-sweetened foods and beverages because of its lower effect on postprandial blood sugar levels, and also noted that 'high intakes of fructose may lead to metabolic complications such as dyslipidaemia, insulin resistance, and increased visceral adiposity'. Further, the UK’s Scientific Advisory Committee on Nutrition in 2015 disputed the claims of fructose causing metabolic disorders, stating that 'there is insufficient evidence to demonstrate that fructose intake leads to adverse health outcomes independent of any effects related to its presence as a component of total and free sugars.' The word 'fructose' was coined in 1857 from the Latin for fructus (fruit) and the generic chemical suffix for sugars, -ose. It is also called fruit sugar and levulose. Fructose is a 6-carbon polyhydroxyketone. Crystalline fructose adopts a cyclic six-membered structure owing to the stability of its hemiketal and internal hydrogen-bonding. This form is formally called d-fructopyranose. In water solution, fructose exists as an equilibrium mixture of 70% fructopyranose and about 22% fructofuranose, as well as small amounts of three other forms, including the acyclic structure. Fructose may be anaerobically fermented by yeast or bacteria. Yeast enzymes convert sugar (glucose, or fructose) to ethanol and carbon dioxide. The carbon dioxide released during fermentation will remain dissolved in water, where it will reach equilibrium with carbonic acid, unless the fermentation chamber is left open to the air. The dissolved carbon dioxide and carbonic acid produce the carbonation in bottled fermented beverages. Fructose undergoes the Maillard reaction, non-enzymatic browning, with amino acids. Because fructose exists to a greater extent in the open-chain form than does glucose, the initial stages of the Maillard reaction occur more rapidly than with glucose. Therefore, fructose has potential to contribute to changes in food palatability, as well as other nutritional effects, such as excessive browning, volume and tenderness reduction during cake preparation, and formation of mutagenic compounds. Fructose readily dehydrates to give hydroxymethylfurfural ('HMF').This process, in the future, may become part of a low-cost, carbon-neutral system to produce replacements for petrol and diesel from plants. The primary reason that fructose is used commercially in foods and beverages, besides its low cost, is its high relative sweetness. It is the sweetest of all naturally occurring carbohydrates. The relative sweetness of fructose has been reported in the range of 1.2–1.8 times that of sucrose. However, it is the 6-membered ring form of fructose that is sweeter; the 5-membered ring form tastes about the same as usual table sugar. Warming fructose leads to formation of the 5-membered ring form. Therefore, the relative sweetness decreases with increasing temperature. However it has been observed that the absolute sweetness of fructose is identical at 5 °C as 50 °C and thus the relative sweetness to sucrose is not due to anomeric distribution but a decrease in the absolute sweetness of sucrose at lower temperatures.