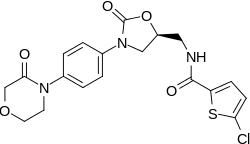
Rivaroxaban

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication (blood thinner) used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth. Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication (blood thinner) used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth. Common side effects include bleeding. Other serious side effects may include spinal hematoma and anaphylaxis. It is unclear if use in pregnancy and breastfeeding is safe. Compared to warfarin it has fewer interactions with other medications. It works by blocking the activity of the clotting protein factor Xa. Rivaroxaban was patented in 2007 and approved for medical use in the United States in 2011. In the United States, it will not be available as a generic medication until 2024. A month supply in the United Kingdom costs the NHS about 50 £ as of 2019. In the United States, the wholesale cost of this amount is about 430 USD. In 2016, rivaroxaban was the 105th most prescribed medication in the United States with more than 7 million prescriptions. In those with non-valvular atrial fibrillation, it appears to be as effective as warfarin in preventing ischemic strokes and embolic events. Rivaroxaban is associated with lower rates of serious and fatal bleeding events than warfarin, though rivaroxaban is associated with higher rates of bleeding in the gastrointestinal tract. In July 2012, the UK's National Institute for Health and Clinical Excellence recommended rivaroxaban to prevent and treat venous thromboembolism. Because of the difficulty associated with managing bleeding, rivaroxaban should be discontinued at least 24 hours before surgery, then restarted as soon as adequate hemostasis is established. Current dosing recommendations do not recommended administering rivaroxaban with drugs known to be strong combined CYP3A4/P-glycoprotein inhibitors because this results in significantly higher plasma concentrations of rivaroxaban. The most serious adverse effect is bleeding, including severe internal bleeding. Rivaroxaban is associated with lower rates of serious and fatal bleeding events than warfarin but is associated with higher rates of bleeding in the gastrointestinal tract. While a reversal agent for rivaroxaban is now available (Andexanet alfa/AndexXa); its safety and efficacy are not as well established as the reversal agents for the older anticoagulant, warfarin (vitamin K and prothrombin complex concentrate), meaning that serious bleeding may be more difficult to manage. As of 2015, post-marketing assessments showed liver toxicity, and further studies are needed to quantify this risk. The medication is contraindicated in people with significant liver disease and end-stage kidney disease, in whom the medication was not trialed.