TEAD1

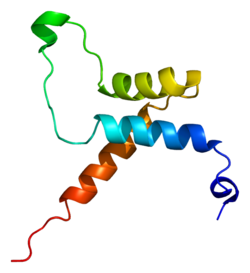
2HZD, 3KYS, 4RE1, 4Z8E700321676ENSG00000187079ENSMUSG00000055320P28347P30051NM_021961NM_001166584NM_001166585NM_009346NM_175559NP_068780NP_001160056NP_001160057NP_033372Transcriptional enhancer factor TEF-1 also known as TEA domain family member 1 (TEAD1) and transcription factor 13 (TCF-13) is a protein that in humans is encoded by the TEAD1 gene. TEAD1 was the first member of the TEAD family of transcription factors to be identified.2hzd: NMR structure of the DNA-binding TEA domain and insights into TEF-1 function Transcriptional enhancer factor TEF-1 also known as TEA domain family member 1 (TEAD1) and transcription factor 13 (TCF-13) is a protein that in humans is encoded by the TEAD1 gene. TEAD1 was the first member of the TEAD family of transcription factors to be identified. All members of the TEAD family share a highly conserved DNA binding domain called the TEA domain. This DNA binding domain has a consensus DNA sequence 5’-CATTCCA/T-3’ that is called the MCAT element. The three dimensional structure of the TEA domain has been identified . Its conformation is close to that of the homeodomain and contains 3 α helixes (H1, H2 and H3). It is the H3 helix that enables TEAD proteins to bind DNA. Another conserved domain of TEAD1 is located at the C terminus of the protein. It allows the binding of cofactors and has been called the YAP1 binding domain, because it is its ability to bind this well-known TEAD proteins co-factor that led to its identification. Indeed, TEAD proteins cannot induce gene expression on their own. They have to associate with cofactors to be able to act TEAD1 is expressed in various tissues including skeletal muscle, pancreas, placenta, lung, and heart. TEAD proteins are found in many organisms under different names, assuming different functions. For example, in Saccharomyces cerevisiae TEC-1 regulates the transposable element TY1 and is involved in pseudohyphale growth (the elongated shape that yeasts take when grown in nutrient-poor conditions). In Aspergillus nidulans, the TEA domain protein ABAA regulates the differentiation of conidiophores. In drosophila the transcription factor Scalloped is involved in the development of the wing disc, survival and cell growth. Finally in Xenopus it has been demonstrated that the ortholog of TEAD1 regulates muscle differentiation. Protein Kinase A (pKA) can phosphorylate TEAD1 at serine 102, after the TEA domain. This phosphorylation is needed for the transcriptional activation of the α MyHC gene. Protein Kinase C (pKC) phosphorylates TEAD1 on serine and threonine next to the last alpha loop in the TEA domain. This phosphorylation decreases TEAD1 binding to the GTIIC enhancer.TEAD1 can be palmitoylated on a conserved cysteine at the C-term of the protein. This post-translational modification is critical for proper folding of TEAD proteins and their stability. TEAD proteins require cofactors to induce the transcription of target genes. TEAD1 interacts with all members of the SRC family of steroid receptor coactivators. In HeLa cells TEAD1 and SRC induce gene expression, TEAD1 interacts with PARP (Poly-ADP ribose polymerase) to regulate smooth muscle α-actin expression. PARP can also ADP-ribosylate the TEAD proteins and make the chromatin context favorable to transcription through histone modification, SRF (Serum response factor) and TEAD1 together regulate gene expression. TEAD proteins and MEF2 (myocyte enhancer factor 2) interact physically. The binding of MEF2 on DNA induces and potentiates TEAD1 recruitment at MCAT sequences that are adjacent to MEF2 binding sites. This recruitment leads to the repression of the MLC2v (Myosin Light Chain 2 v) and βMHC ( β-myosin heavy chain ) promoter. TEAD1 and the phosphoprotein MAX interact in vivo and in vitro. Once this complex is formed, these two proteins can regulate the alpha-myosin heavy chain (α-MHC) gene expression. The four Vestigial-like (VGLL) proteins are able to interact with all TEADs. The precise function of TEAD and VGLL interaction is still poorly understood. It has been shown that TEAD/VGLL1 complexes promote anchorage-independent cell proliferation in prostate cancer cell lines suggesting a role in cancer progression Moreover, VGLL2 interaction with TEAD1 activates muscle promoter upon C2C12 differentiation and enhances MyoD-mediated myogenic in 10T1/2. Finally the complex TEAD/VGLL4 acts as a default transcriptional repressor.