Pyruvate decarboxylase

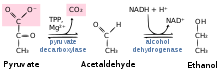
Pyruvate decarboxylase is a homotetrameric enzyme (EC 4.1.1.1) that catalyses the decarboxylation of pyruvic acid to acetaldehyde and carbon dioxide in the cytoplasm of prokaryotes, and in the cytoplasm and mitochondria of eukaryotes. It is also called 2-oxo-acid carboxylase, alpha-ketoacid carboxylase, and pyruvic decarboxylase. In anaerobic conditions, this enzyme is part of the fermentation process that occurs in yeast, especially of the genus Saccharomyces, to produce ethanol by fermentation. It is also present in some species of fish (including goldfish and carp) where it permits the fish to perform ethanol fermentation (along with lactic acid fermentation) when oxygen is scarce. Pyruvate decarboxylase starts this process by converting pyruvate into acetaldehyde and carbon dioxide. Pyruvate decarboxylase depends on cofactors thiamine pyrophosphate (TPP) and magnesium. This enzyme should not be mistaken for the unrelated enzyme pyruvate dehydrogenase, an oxidoreductase (EC 1.2.4.1), that catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA.Cartoon diagram of pyruvate decarboxylase monomer with TPP attached.Cartoon diagram of pyruvate decarboxylase tetramer.Active site of pyruvate decarboxylase with selected amino acids: Glu-51, Glu-477, Asp-444, and Asp-28. Also displayed are cofactors TPP and Mg2+.Positions of His and Cys residues in respect to active site (TPP and Mg) that participate in conformation changes when interacting with pyruvate substrate. Pyruvate decarboxylase is a homotetrameric enzyme (EC 4.1.1.1) that catalyses the decarboxylation of pyruvic acid to acetaldehyde and carbon dioxide in the cytoplasm of prokaryotes, and in the cytoplasm and mitochondria of eukaryotes. It is also called 2-oxo-acid carboxylase, alpha-ketoacid carboxylase, and pyruvic decarboxylase. In anaerobic conditions, this enzyme is part of the fermentation process that occurs in yeast, especially of the genus Saccharomyces, to produce ethanol by fermentation. It is also present in some species of fish (including goldfish and carp) where it permits the fish to perform ethanol fermentation (along with lactic acid fermentation) when oxygen is scarce. Pyruvate decarboxylase starts this process by converting pyruvate into acetaldehyde and carbon dioxide. Pyruvate decarboxylase depends on cofactors thiamine pyrophosphate (TPP) and magnesium. This enzyme should not be mistaken for the unrelated enzyme pyruvate dehydrogenase, an oxidoreductase (EC 1.2.4.1), that catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA. Pyruvate decarboxylase occurs as a dimer of dimers with two active sites shared between the monomers of each dimer. The enzyme contains a beta-alpha-beta structure, yielding parallel beta-sheets. It contains 563 residue subunits in each dimer; the enzyme has strong intermonomer attractions, but the dimers loosely interact to form a loose tetramer. This enzyme is a homotetramer, and therefore has four active sites. The active sites are inside a cavity in the core of the enzyme where hydrogen bonding can occur and where the pyruvate reacts with TPP. Each active site has 20 amino acids, including the acidic Glu-477 (contributes to the stability of the TPP ring) and Glu-51 (aids in cofactor binding). These Glutamates also contribute to forming the TPP ylid, acting as proton donators to the TPP aminopyrimidine ring. The microenvironment around this Glu 477 is very nonpolar, contributing to a higher than normal pKa (normal Glu and Asp pKa's are around 4.6 in small proteins). The lipophilic residues Ile-476, Ile-480 and Pro-26 contribute to the nonpolarity of the area around Glu-477. The only other negatively charged residue apart from TPP coenzyme is the Asp-28, which also aids in increasing the pKa of Glu-477. Thus, the environment of the enzyme must allow for the protonation of the gamma-carboxyl group of Glu-477 to be around pH 6. The aminopyrimidine ring on TPP acts as a base, once in its imine form, to pull off the C2 proton from TPP to form the nucleophile ylide. This must occur because the enzyme has no basic side chains present to deprotonate the TPP C2. A mutation at the active site involving these Glu can result in the inefficiency or inactivity of the enzyme. This inactivity has been proven in experiments in which either the N1' and/or 4'-amino groups are missing. In NMR analysis, it has been determined that when TPP is bound to the enzyme along with the substate-analog pyruvamide, the rate of ylid formation is greater than the normal enzyme rate. Also, the rate of mutation of Glu 51 to Gln reduces this rate significantly. Also included are Asp-444 and Asp-28 which stabilize the active site. These act as stabilizers for the Mg2+ ion that is present in each active site. To ensure that only pyruvate binds, two Cys-221 (more than 20 Ångstroms away from each site) and His-92 trigger a conformational change which inhibits or activates the enzyme depending on the substrate that interacts with it. If the substrate bound in the active site is pyruvate, then the enzyme is activated by a conformational change in this regulatory site. The conformational change involves a 1,2 nucleophilic addition. This reaction, the formation of a thioketal, transforms the enzyme from its inactive to active state. Inhibition of the site is done by a XC6H4CH=CHCOCOOH class of inhibitors/substrate analogues, as well as by the product of decarboxylation from such compounds as cinnamaldehydes. Other potential nucleophilic sites for the inhibitor include Cys-152, Asp-28, His-114, His-115, and Gln-477. The normal catalytic rate of pyruvate decarboxylase is kcat = 10 s−1. However, the rate of the enzyme with a Glu-51 mutation to Gln is 1.7 s−1. The cofactor TPP, C12 H18 N4 O7 P2 S, is needed for this reaction's mechanism; it acts as the prosthetic group to the enzyme. The carbon atom between the sulfur and nitrogen atoms on thiazole ring act as carbanion which binds to the pyruvate.TPP has an acidic H+ on its C2 that acts as the functional part of the thiazolium ring; the ring acts as an 'electron sink', enabling the carbanion electrons to be stabilized by resonance. The TPP can then act as a nucleophile with the loss of this C2 hydrogen, forming the ylide form of TPP. This ylide can then attack pyruvate, which is held by the enzyme pyruvate decarboxylase. During the decarboxylation of pyruvate, the TPP stabilizes the carbanion intermediates as an electrophile by noncovalent bonds. Specifically, the pyridyl nitrogen N1' and the 4'-amino group of TPP are essential for the catalytic function of the enzyme-TDP complex.