Tocopherol

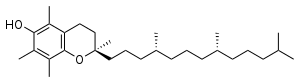
Tocopherols (/toʊˈkɒfəˌrɒl/; TCP) are a class of organic chemical compounds (more precisely, various methylated phenols), many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, it was given the name 'tocopherol' from the Greek words 'τόκος' , and 'φέρειν', meaning in sum 'to carry a pregnancy,' with the ending '-ol' signifying its status as a chemical alcohol. Tocopherols (/toʊˈkɒfəˌrɒl/; TCP) are a class of organic chemical compounds (more precisely, various methylated phenols), many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, it was given the name 'tocopherol' from the Greek words 'τόκος' , and 'φέρειν', meaning in sum 'to carry a pregnancy,' with the ending '-ol' signifying its status as a chemical alcohol. α-Tocopherol is the main source found in supplements and in the European diet, where the main dietary sources are olive and sunflower oils, while γ-tocopherol is the most common form in the American diet due to a higher intake of soybean and corn oil. Tocotrienols, which are related compounds, also have vitamin E activity. All of these various derivatives with vitamin activity may correctly be referred to as 'vitamin E'. Tocopherols and tocotrienols are fat-soluble antioxidants but also seem to have many other functions in the body. Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain which allows for penetration into biological membranes. Both the tocopherols and tocotrienols occur in α (alpha), β (beta), γ (gamma) and δ (delta) forms, determined by the number and position of methyl groups on the chromanol ring. The tocotrienols have the same methyl structure at the ring and the same Greek letter-methyl-notation, but differ from the analogous tocopherols by the presence of three double bonds in the hydrophobic side chain. The unsaturation of the tails gives tocotrienols only a single stereoisomeric carbon (and thus two possible isomers per structural formula, one of which occurs naturally), whereas tocopherols have 3 centers (and eight possible stereoisomers per structural formula, again, only one of which occurs naturally). Each form has a different biological activity.In general, the unnatural l-isomers of tocotrienols lack almost all vitamin activity, and half of the possible 8 isomers of the tocopherols (those with 2S chirality at the ring-tail junction) also lack vitamin activity. Of the stereoisomers which retain activity, increasing methylation, especially full methylation to the alpha-form, increases vitamin activity. In tocopherols, this is due to the preference of the tocopherol binding protein for the alpha-tocopherol form of the vitamin. As a food additive, tocopherol is labeled with these E numbers: E306 (tocopherol), E307 (α-tocopherol), E308 (γ-tocopherol), and E309 (δ-tocopherol). These are all approved in the USA, EU and Australia and New Zealand for use as antioxidants. Alpha-tocopherol is the form of vitamin E that is preferentially absorbed and accumulated in humans. The measurement of 'vitamin E' activity in international units (IU) was based on fertility enhancement by the prevention of miscarriages in pregnant rats relative to alpha-tocopherol.