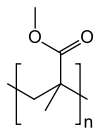
Poly(methyl methacrylate)

Poly(methyl methacrylate) (PMMA), also known as acrylic, acrylic glass, or plexiglass as well as by the trade names Crylux, Plexiglas, Acrylite, Lucite, and Perspex among several others (see below), is a transparent thermoplastic often used in sheet form as a lightweight or shatter-resistant alternative to glass. The same material can be used as a casting resin, in inks and coatings, and has many other uses.High heel shoes made of LuciteAn electric bass guitar made from poly(methyl methacrylate) Poly(methyl methacrylate) (PMMA), also known as acrylic, acrylic glass, or plexiglass as well as by the trade names Crylux, Plexiglas, Acrylite, Lucite, and Perspex among several others (see below), is a transparent thermoplastic often used in sheet form as a lightweight or shatter-resistant alternative to glass. The same material can be used as a casting resin, in inks and coatings, and has many other uses. Although not a type of familiar silica-based glass, the substance, like many thermoplastics, is often technically classified as a type of glass (in that it is a non-crystalline vitreous substance) hence its occasional historical designation as acrylic glass. Chemically, it is the synthetic polymer of methyl methacrylate. The material was developed in 1928 in several different laboratories by many chemists, such as William Chalmers, Otto Röhm, and Walter Bauer, and was first brought to market in 1933 by German Röhm & Haas AG (as of January 2019 part of Evonik Industries) and its partner and former U.S. affiliate Rohm and Haas Company under the trademark Plexiglas. PMMA is an economical alternative to polycarbonate (PC) when tensile strength, flexural strength, transparency, polishability, and UV tolerance are more important than impact strength, chemical resistance and heat resistance. Additionally, PMMA does not contain the potentially harmful bisphenol-A subunits found in polycarbonate. It is often preferred because of its moderate properties, easy handling and processing, and low cost. Non-modified PMMA behaves in a brittle manner when under load, especially under an impact force, and is more prone to scratching than conventional inorganic glass, but modified PMMA is sometimes able to achieve high scratch and impact resistance. The first acrylic acid was created in 1843. Methacrylic acid, derived from acrylic acid, was formulated in 1865. The reaction between methacrylic acid and methanol results in the ester methyl methacrylate. Polymethyl methacrylate was discovered in the early 1930s by British chemists Rowland Hill and John Crawford at Imperial Chemical Industries (ICI) in England. ICI registered the product under the trademark Perspex. About the same time, chemist and industrialist Otto Röhm of Rohm and Haas AG in Germany attempted to produce safety glass by polymerizing methyl methacrylate between two layers of glass. The polymer separated from the glass as a clear plastic sheet, which Röhm gave the trademarked name Plexiglas in 1933. Both Perspex and Plexiglas were commercialized in the late 1930s. In the United States, E.I. du Pont de Nemours & Company (now DuPont Company) subsequently introduced its own product under the trademark Lucite. In 1936 ICI Acrylics (now Lucite International) began the first commercially viable production of acrylic safety glass. During World War II both Allied and Axis forces used acrylic glass for submarine periscopes and aircraft windshields, canopies, and gun turrets. Airplane pilots whose eyes were damaged by flying shards of PMMA fared much better than those injured by standard glass, demonstrating better compatibility between human tissue and PMMA than glass. Civilian applications followed after the war. Common orthographic stylings include polymethyl methacrylate and polymethylmethacrylate. The full IUPAC chemical name is poly(methyl 2-methylpropenoate). (It is a common mistake to use 'an' instead of 'en'.) Although PMMA is often called simply 'acrylic', acrylic can also refer to other polymers or copolymers containing polyacrylonitrile. Notable trade names include Acrylite, Lucite, R-Cast, Plexiglas, Optix, Perspex, Oroglas, Altuglas, Cyrolite, and Sumipex. PMMA is routinely produced by emulsion polymerization, solution polymerization, and bulk polymerization. Generally, radical initiation is used (including living polymerization methods), but anionic polymerization of PMMA can also be performed. To produce 1 kg (2.2 lb) of PMMA, about 2 kg (4.4 lb) of petroleum is needed. PMMA produced by radical polymerization (all commercial PMMA) is atactic and completely amorphous. The glass transition temperature (Tg) of atactic PMMA is 105 °C (221 °F). The Tg values of commercial grades of PMMA range from 85 to 165 °C (185 to 329 °F); the range is so wide because of the vast number of commercial compositions which are copolymers with co-monomers other than methyl methacrylate. PMMA is thus an organic glass at room temperature; i.e., it is below its Tg. The forming temperature starts at the glass transition temperature and goes up from there. All common molding processes may be used, including injection molding, compression molding, and extrusion. The highest quality PMMA sheets are produced by cell casting, but in this case, the polymerization and molding steps occur concurrently. The strength of the material is higher than molding grades owing to its extremely high molecular mass. Rubber toughening has been used to increase the toughness of PMMA to overcome its brittle behavior in response to applied loads. PMMA can be joined using cyanoacrylate cement (commonly known as superglue), with heat (welding), or by using chlorinated solvents such as dichloromethane or trichloromethane (chloroform) to dissolve the plastic at the joint, which then fuses and sets, forming an almost invisible weld. Scratches may easily be removed by polishing or by heating the surface of the material.