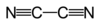Cyanogen

Cyanogen is the chemical compound with the formula (CN)2. It is a colorless, toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C−C≡N, although other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr). Cyanogen is the chemical compound with the formula (CN)2. It is a colorless, toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C−C≡N, although other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr). Cyanogen is the anhydride of oxamide: although oxamide is manufactured from cyanogen by hydrolysis: Cyanogen is typically generated from cyanide compounds. One laboratory method entails thermal decomposition of mercuric cyanide: Alternatively, one can combine solutions of copper(II) salts (such as copper(II) sulfate) with cyanides, an unstable copper(II) cyanide is formed which rapidly decomposes into copper(I) cyanide and cyanogen. Industrially, it is created by the oxidation of hydrogen cyanide, usually using chlorine over an activated silicon dioxide catalyst or nitrogen dioxide over a copper salt. It is also formed when nitrogen and acetylene are reacted by an electrical spark or discharge.