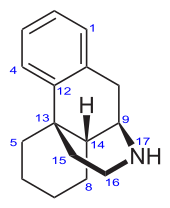
Morphinan

Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Morphinan is the prototype chemical structure of a large chemical class of psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others. Morphinan has a phenanthrene core structure with the A ring remaining aromatic and the B and C rings being saturated, and an additional nitrogen-containing, six-membered, saturated ring, the D ring, being attached to carbons 9 and 13 of the core, and with the nitrogen being at position 17 of the composite. Of the major naturally occurring opiates of the morphinan type—morphine, codeine and thebaine—thebaine has no therapeutic properties (it causes seizures in mammals), but it provides a low-cost feedstock for the industrial production of at least four semi-synthetic opiate agonists, including hydrocodone, hydromorphone, oxycodone and oxymorphone, and, perhaps more significantly, the opioid antagonist naloxone. The physiological behavior of morphinans (naturally occurring and semi-synthetic derivatives) is thought to be associated with the aromatic A ring, the nitrogen-containing D ring and the 'bridge' between these two rings formed by carbons 9, 10 and 11 of the core, with the D ring 'above' the core (levorotatory).