Metallothionein

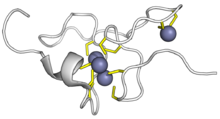
Metallothionein (MT) is a family of cysteine-rich, low molecular weight (MW ranging from 500 to 14000 Da) proteins. They are localized to the membrane of the Golgi apparatus. MTs have the capacity to bind both physiological (such as zinc, copper, selenium) and xenobiotic (such as cadmium, mercury, silver, arsenic) heavy metals through the thiol group of its cysteine residues, which represent nearly 30% of its constituent amino acid residues.X-C-X(20)-CSCGAXCNCASC-X(3,5) Metallothionein (MT) is a family of cysteine-rich, low molecular weight (MW ranging from 500 to 14000 Da) proteins. They are localized to the membrane of the Golgi apparatus. MTs have the capacity to bind both physiological (such as zinc, copper, selenium) and xenobiotic (such as cadmium, mercury, silver, arsenic) heavy metals through the thiol group of its cysteine residues, which represent nearly 30% of its constituent amino acid residues. MT was discovered in 1957 by Vallee and Margoshe from purification of a Cd-binding protein from horse (equine) renal cortex. MT plays a role in the protection against metal toxicity and oxidative stress, and is involved in zinc and copper regulation. There are four main isoforms expressed in humans (family 1, see chart below): MT1 (subtypes A, B, E, F, G, H, L, M, X), MT2, MT3, and MT4. In the human body, large quantities are synthesised primarily in the liver and kidneys. Their production is dependent on availability of the dietary minerals such as zinc, copper, and selenium, as well as the amino acids histidine and cysteine. Metallothioneins are rich in thiols, causing them to bind a number of trace metals. Metallothionein binds several Zn ions. One of few eukaryotic proteins distinguished as having a role in substantial metal detoxification. Zinc and Cadmium are tetrahedrally coordinated to cysteine residues, each metallothionein protein molecule may bind up to 7 atoms of Zn or Cd. The biosynthesis of metallotionein appeared to have increased by several-fold throughout oxidative stress to shield the cells against cytotoxicity and DNA damage. Metallothionein biosynthesis can also be induced by certain agents or conditions, for example, hormones, pharmaceuticals, alcohols, other substance treatments and many more. Metallothionein is a cytoplasmic protein, in an adult liver, it is localized mainly in the cytoplasm. In human fetus, metallothionein is localized in hepatocyte nuclei. MTs are present in a vast range of taxonomic groups, ranging from prokaryotes and in eukaryotes (such as the cyanobacteria Synechococcus sp.), protozoa (such as the ciliate Tetrahymena genera), plants (such as Pisum sativum, Triticum durum, Zea mays, or Quercus suber), yeast (such as Saccharomyces cerevisiae or Candida albicans), invertebrates (such as the nematode Caenorhabditis elegans, the insect Drosophila melanogaster, the mollusc Mytilus edulis, or the echinoderm Strongylocentrotus purpuratus) and vertebrates (such as the chicken Gallus gallus, or the mammalian Homo sapiens or Mus musculus).